Background on Biofilms
Biofilms are one of the most significant survival mechanisms microbes have to survive in ecosystems. They are also critical to our Earth’s ecosystems both on the macro and micro scales for organic nutrient, mineral deposition and utilization in ecological cycles. A biofilm truly is a “microbial forest” that enables a number of bacterial species to resist predation by other microbes like protozoa while enduring harsh environmental conditions and cycles.
Like forests, biofilms are dynamic with various phases of establishing, populating and growing this forest with impacts upon other microbes and biological entities. The past 20 years in biofilm study and research has given us an appreciation of the dynamic, complex entity that they are.
Biofilm Formation in Food Plants
Formation in food plants is a dynamic and vital function in order for either beneficial or harmful bacteria to survive. Consider a food plant to be a dynamic ecosystem on steroids. The whole adaptation of biofilm formation, destruction or damage, and renewal is very accelerated in a typical food plant due to the nature of food processing, and food plant sanitation and environmental hygiene.
Biofilm-producing microbes have adapted to food plant environs based on a number of criteria. These criteria include ingredient and product matrix, plant design and its environmental deficiencies, and processing line and equipment designs coupled with the adverse control measures these biofilm-producing microbes encounter. Some spoilage/pathogenic microbes may not be the direct biofilm producers, but have developed a symbiotic relationship with the actual biofilm-producing strains. In other plant dynamics, this occurs when spoilage and/pathogenic strains have adapted to rapidly create biofilms.
This accelerated process still includes the same dynamics found in nature. After “proper” sanitation, all vestiges of biofilm have been removed from either a food contact or environmental surface. Native microflora in a plant’s ecosystem look to restore its survivability by seeking to form a biofilm. Food ingredients and usually water/moisture (except for a properly-maintained dry processing operation) are introduced immediately during production.
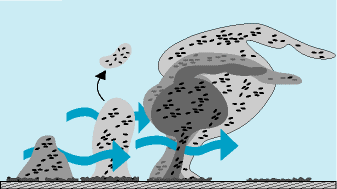
Source: Montana State, Center for Biofilm Engineering
The diagram insert clearly shows the phases of biofilm formation. After a conditioning phase, specific genes in bacteria using their surface appendages (flagella, fimbriae, and pili) secrete the biofilm foundation chemistry, the EPS (Extracellular Polysaccharides). The EPS or “slime” is what forms the “living cement” that helps to build the biofilm making it increasingly difficult to remove.
By 8 to 12 hours, if no sanitation has occurred, the third phase of biofilm growth, and maturation begins. EPS is copiously produced now, protecting the very cells that are producing the biofilm and also the visiting spoilage and pathogenic strains that contribute benefits to this microbial community. Even up to 24 hours, these biofilms are considered to be young or immature.
However, as time progresses with no sanitation step, the biofilm undergoes continual growth, complexity, and the dynamics of sloughing occurs. This is particularly found in food plants on environmental surfaces which will not be typically cleaned and sanitized in a 24-hour time frame. The sloughing of cells found in water lines, AC drip pans, drains, etc., can and will create the sporadic spikes in environmental swabbing, food contact swabbing or product samples. Sloughed cells are also the way the biofilm spreads and grows in a plant ecosystem. The small sloughed biofilm fragments re-attach elsewhere, and start a new larger biofilm community either in a non-food contact surface, equipment component or in poorly cleaned equipment.
Impact of Biofilms upon Food Safety Programs
Of course, in many instances the microbes within undetected or untreated biofilms on environmental or food contact surfaces that cross-contaminate products or ingredients are spoilage microbes that can adversely affect food quality. However, for a HACCP program, food safety can be adversely impacted by those biofilm-containing pathogens, particularly those having Salmonella spp., Listeria monocytogenes, E. coli or the Bacillus species.
Due to the ubiquity and hardiness of Listeria monocytogenes (Lm), a lot of studies have been undertaken on how Lm strains can form biofilms. Later, in control measures below, we will site some interesting studies upon how these control modalities can be successively implemented.
The surface composition is a key criterion as to how readily and tenaciously a biofilm can form either on a food contact or environmental surface. Porous surfaces like cinder block, granite or marble readily form Listeria-containing biofilms and are more difficult to remove. While 304 or 316 grades of stainless steel are considered good, optimal surfaces to remove biofilms, if there are improper sanitation procedures or hygienic design, Listerial-based biofilms will readily form. [1]
Conveyor belts, being direct or indirect food contact zones in most cases, are also critical for control of biofilm formation and their removal. A recent study by Texas Tech using Listerial biofilms showed that acetal or polypropylene mesh-type belts had the best biofilm formation rates, less so with a non-weave type. While stainless steel in this study had the least, it depended upon the surface area of the belt with a weave having faster and more tenacious biofilm formation than a single loop stainless belt. [2]. Consequently, any surface type or design that’s conducive to biofilm formation is also more difficult to remove or control that biofilm from forming.
It is also critical that the hygienic design of the processing equipment not have voids and crevices that make proper biofilm removal difficult, if not impossible. Some older, or worn equipment, must either be replaced or refurbished to remove the growth niches due to poor hygienic design.
Biofilm Control Measures for Food Safety
The key control measure is the plants overall sanitation program for both environmental and food contact surfaces. This must be comprehensive, for not only cross-contamination vectors derived from direct and indirect food contact zones (1 & 2), but from adjacent environmental vectors like drains, flooring, walls, air handling units including drip pans, and overhead fixtures. (Zones 3 & 4)
Since a biofilm’s EPS utilizes a cocktail of physical and chemical bonding interactions to adhere to and protect that adhesion to a surface, this employs a lot of potential energy to create and to overcome. Consequently, in order to remove that adolescent or mature biofilm, you need an appropriate level of energies to remove that biofilm. This requires thermal energy (rinse water temperature or surface temperature), mechanical energy (rinse water pressure), and mechanical agitation like scrubbing.
It also requires the appropriate chemical energy within the properly-selected cleaner to assist in removing that biofilm. For example, since the immature or mature biofilm contains carbohydrates, and proteins, you usually need to utilize a chlorinated-alkaline product delivered either as a foam or gel product for non- pipeline open-surface sanitation. The same type of formulated product in a low- foaming formulation is needed for a true closed pipeline-type CIP system or for a COP tank. COP tanks are an excellent approach to remove biofilms from small parts like pipe or valve fitting, gaskets and O-rings.
Unfortunately, in some instances either the SSOP overlooks the proper application of the appropriate mechanical energies to assist in stripping the biofilm from its surface, or the sanitation operator is pressed for time and relies on the chemical energy or an easy mechanical energy venue like high-pressure rinsing to remove the biofilm. While high-pressure has a role to assist in biofilm removal with problematic equipment, it can also create dangerous pathogen containing aerosols that re-contaminate equipment while also damaging that equipment item.
Other forms of mechanical energies or agitation need to be applied to reduce the need for high-pressure removal of the biofilms like non-abrasive scrub pads or soft bristle nylon brushes (manual) or automated systems, like automated belt cleaning nylon rotary brushes, or Brite Belt pads.
In some instances, you can not properly or efficiently apply even a CIP cleaner to attain the proper minimal flow velocity of 5 ft /sec for water or food pipelines. Utilization of an approved disinfectant concentration of an oxidizing biocide like Peracetic acid [liquid or foam versions] or Chlorine dioxide solutions, are best modalities for “purge” disinfection flushes of pipeline systems. [3] These, of course, are followed by a water flush that can contain approved food contact sanitizer use concentration levels. The maturity and severity of the biofilm in these pipeline systems will dictate both the use concentration range, and the duration of the application.
There are other modalities being utilized both to remove and assist in controlling the rate of biofilm formation on food contact or even environmental surfaces. Use of ultrasonication during operation on a critical food contact surface and during the sanitation procedure have been found to remove more soil, biofilm, and microbes like Lm than sanitation without the ultrasonication. [4] Even for drain sanitation, the use of ultrasonication in concert with foaming Peracetic acid has shown marked improvement in removing Lm-based biofilms in PVC drain pipes. [5] Furthermore, other studies by equipment manufacturers employing coating surfaces with silver halides or other surfaces with polyethylene films containing amines that get charged by chlorine sanitizers have been shown to reduce adhesion, and slow biofilm formation. [6]
Also, utilization of automated foamed QAC or Sterilex on flooring and drains for wet applications coupled with pellet or powder-based QAC or Hydrogen Peroxide systems can markedly control biofilm formation, especially Lm-containing biofilms on floors and drains in RTE food plants. [7]
Through a coordinated program of proper equipment or plant hygienic design, coupled with the appropriate sanitation procedures using the appropriate chemistry, and physical/mechanical agitation approaches, we can control and slow down biofilm from forming on both food contact and environmental surfaces.
References
[1] Silva, et al., Journal Food Protection, Vol. 71 (7) Nov. 2008, pg 1379-1385, Adhesion to and Viability of Listeria monocytogenes on Food Contact Surfaces.
[2] Pitchiah, et al., Food Protection Trends, Vol. 28, Nov. 2008, Biofilm Formation of Listeria monocytogenes on various Conveyor belt surfaces.
[3] Martin, et al., Journal Food Protection, Vol. 68 (3) March 2005, p 494-498, Elimination of Listeria monocytogenes Biofilms by Ozone, Chlorine & Hydrogen Peroxide.
[4] Lunden, et al., Journal Food Protection, Vol. 72 (2) February 2009, p 408-411, Pilot Scale Continuous Ultrasonic Cleaning Equipment Reduces Listeria monocytogenes levels on Conveyor belts.
[5] Berrang, et al, Journal Food Protection, Vol. 71 (1) January 2008, p 66-69. Effect of Chemical Sanitizers with and without Ultrasonication on Listeria monocytogenes as a Biofilm within Polyvinyl Chloride Drain Pipes.
[6] Goddard, & Hotchkiss, Journal Food Protection, Vol. 71 (10), October 2008, p 2042-2047, Rechargeable Antimicrobial Surface Modification of Polyethylene.
[7] Ceylon, Erdogan, Silliker Laboratories. 2011. RPN 15202, July 14, 2011, Validation of Quaternary Ammonia and Hydrogen Peroxide Powder for Control of Listeria monocytogenes in Ready-to-Eat Meat and Poultry Plants.

